Manufacturing
MANUFACTURING
We have built veritable presence with Quality branded generic formulations in more than 70+ Countries across the globe. Our business is preserving and improving human life. The multi located plants conform to current GMP as prescribed by WHO and are managed by highly experienced and committed people. There are dedicated areas for different sections like :
1. Dry powder filling
2. Liquid parenterals
3. Oral formulations (Tablets, Capsules)
4. Ointments
5. Nasal sprays
The plants are manned by highly experienced and energetic production team, which follows strict GMP guidelines including equipment /process validation, backed by detailed documentation that ensures consistent product quality.
MANUFACTURING FACILITY
AT NEON QUALITY PRECEDES ALL CONSIDERATIONS

NEON’s commitment to quality is unswerving and is backed by its team of professionals who are always ready to serve with high quality and efficient services. NEON consistently set new benchmark in quality assurance, while adhering to those imposed by regulatory bodies. The highly trained Quality Assurance/ Quality Control teams are backed by full-fledged laboratory and specialized analytical instruments. The quality system covers elaborated IQ/OQ/PQ validations which have been completed on all applicable equipments. NEON’s credential as a registered drug manufacturing facility with impressive inspection record and its experience in quality documentation has helped to work in tandem with all applicable local state regulations on quality, environmental & safety regulations.
GLOBAL PRESENCE
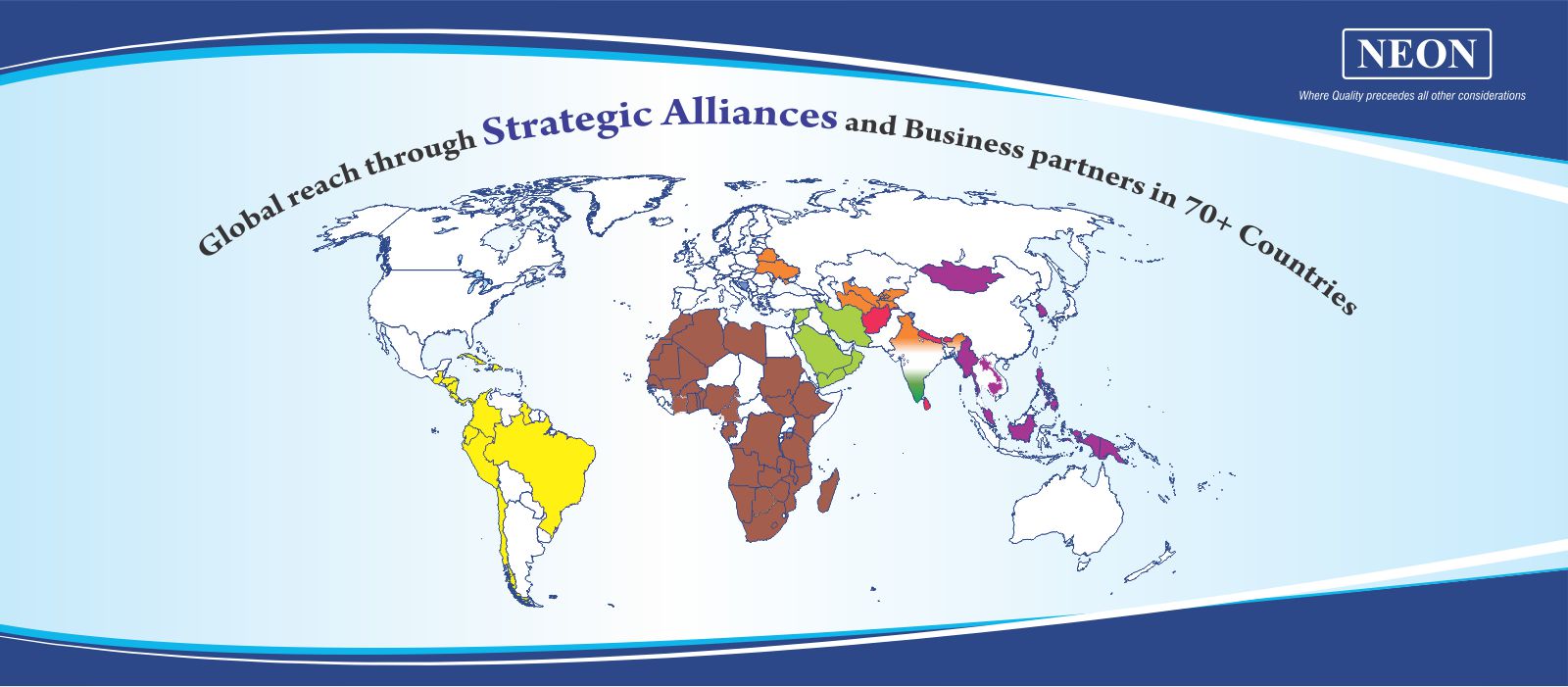
We have multiple subsidiaries and associates catering to various verticals of healthcare domains.
NEON has built a veritable presence in supplying high quality branded generic formulations across the globe. NEON exports its products to more than 70+ countries with more than 461 products registered worldwide.
NEON exports its products to various key markets in Latin America, Africa, Middle East, Europe, South East Asia, CIS & SAARC countries.